Home· · ·Techcomp college· · ·Application notes
Application notes
EI ANT01 - Kinetics of Photocatalysis Reactions Studied by Transient Absorption Spectroscopy
Photocatalysis is the rate increase of a chemical reaction by light, often in the presence of a catalyst that starts the reaction upon irradiation. Photocatalysts are typically semiconducting metal oxides such as ZnO, Fe2O3 or TiO2 which are employed as particles in solution. When absorbing light, these materials are able to generate electrons and holes which go on to react with chemical species on their surface.
The photocatalysis process is illustrated in Figure 1. If a photon of greater energy than the bandgap of the semiconductor is absorbed by the photocatalyst, it excites an electron (e–) from the valence band into the conduction band. This process generates a hole (h+) or positive charge in the valence band. These charge carriers are mobile, albeit short lived, and can evolve through multiple pathways. The electron-hole pair can recombine emitting energy after a short time, but the charge carriers can also be trapped at specific sites in the material. If they do not recombine, electrons and holes can react with species on the surface of the photocatalyst. Valence band electrons can combine with electron acceptors in solution, and conduction band holes may react with electron donors. This is often the first step in a series of charge-transfer reactions in solution that lead to a desired product.
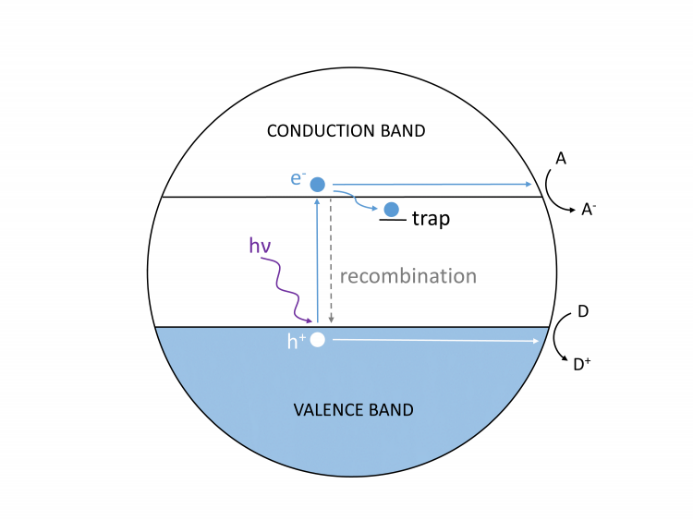
Figure 1: Charge carrier generation in a photocatalyst particle. Conduction band electrons react with electron acceptors (A) and valence band holes react with electron donors (D).
Although photocatalysis has been known since the early 20th century, its first major practical application was not found until 1972 when Honda and Fujishima reported the photocatalytic splitting of water in the presence of titanium dioxide, TiO2.1 Since then TiO2 has established itself as a widely used photocatalyst. Despite its large bandgap requiring UV irradiation to be activated, it has some desirable properties such as high stability, high reactivity and low price.
The fate of the photogenerated carriers in a photocatalyst depends on the material, particle size and the presence of defects. Research on TiO2 and other photocatalysts focuses on increasing their efficiency by varying these parameters. Studying the kinetics of charge carrier transfer and recombination is a basic step in the design of photocatalytic materials: the longer the lifetime of the electron-hole pair, the better their chance of taking part in reactions. Some recombination events proceed via emission of light and can be followed by photoluminescence spectroscopy, however there is a large proportion of nonradiative recombination (i.e. non-emitting) events. For this reason, transient absorption (TA) spectroscopy is often used to follow the evolution of photogenerated charge carriers in TiO2. Charge carrier lifetimes can range from ps to ms, so they should be studied with a TA instrument covering a broad range of timescales. In addition, it is possible to follow the kinetics of photocatalytic reactions by monitoring the transient absorption from reaction intermediates.
In this application note, an Edinburgh Instruments LP980 Transient Absorption Spectrometer (Figure 2) is used to probe the dynamics of photogenerated charge carriers in a suspension of TiO2 particles. A hole-accepting species such as chloride is shown to be oxidised by activated TiO2, and the time-dependent absorbance of the dichloride radical is used to elucidate its reaction kinetics.

Figure 2: Edinburgh Instruments LP980 Transient Absorption Spectrometer



 2606, 26/F., Tower 1, Ever Gain Plaza, 88 Container Port Road, Kwai Chung, N.T., Hong Kong
2606, 26/F., Tower 1, Ever Gain Plaza, 88 Container Port Road, Kwai Chung, N.T., Hong Kong +852-27519488 / WhatsApp/WeChat HK: +852-8491 7250
+852-27519488 / WhatsApp/WeChat HK: +852-8491 7250 techcomp@techcomp.com.hk
techcomp@techcomp.com.hk
 Sweep The Concern Us
Sweep The Concern Us