Home· · ·Techcomp college· · ·Application notes
Application notes
Analysis of Arsenic in Foods by Zeeman Correction Atomic Absorption Spectrophotometer with Hydride F
SUBJECT : ANALYSIS OF ARSENIC IN FOODS BY ZEEMAN ORRECTION ATOMIC ABSORPTION SPECTROPHOTOMETER WITH HYDRIDE FORMATION SYSTEM
INSTRUMENT : MODEL Z-2000 (Z-2300) POLARIZED ZEEMAN FLAME ATOMIC ABSORPTION SPECTROPHOTOMETER WITH MODEL HFS-3 HYDRIDE FORMATION SYSTEM


Hydride Formation System
1. INTRODUCTION
Interest in food safety, has become more focused it is featured in as more newspapers, magazines and other mass media than ever before. Metal elements contained in foods are also drawing attention, and they are regarded as constituents
which should be analyzed regularly. Arsenic (As) is thought to be essential for living organisms. However, things are not so simple, because a low As intake may develop
into deficiency diseases and a high intake may trigger excess symptoms. Therefore, it is necessary to establish an analytical method for As in foods.
Generally, As in foods is measured with an atomic absorption spectrophotometer coupled with a hydride formation system. In this method, the wavelength used for arsenic analysis is short, or 193.7 nm and the sample is atomized in a quartz furnace which is partially heated. This requires that both the optical path for lamp drift compensation (for double-beam optics) and that for background correction (with a deuterium lamp) are required to pass through the quartz furnace. Yet this condition is not met by any of the conventional instruments. Thus noise and drift are apt to
occur in measurement, which calls for improvement on baseline stability.
The Hitachi Model Z-2000 polarized Zeeman atomic absorption spectrophotometer utilizes the Zeeman effect for the quartz-heating hydride formation method for the first time in the world.
In this combination of the Zeeman
effect-based methods of quartz-heating hydride formation and atomic absorption,
all optical paths required for lamp drift compensation and background
correction are passed through the heating quartz furnace on the same optical
axis. Therefore, a stable baseline can be obtained, and noise and drift can be
reduced, thus ensuring a highly reliable analysis. An example application of
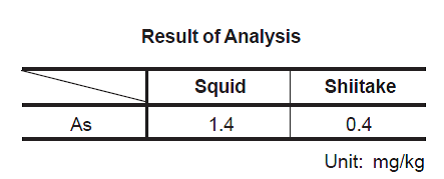
this technique to the analysis of foodstuffs (Shiitake and squid is presented
here).
2. PHOTOMETRIC METHOD
Tre Zeeman correction method is compared with the single beam method. Single Beam Method: When a heatable quartz cell is placed on a magnet, the Zeeman correction effect cannot be used. So, the single beam method is used for measurement. (Noise and drift are likely to be significant. See Data 1.) Zeeman Correction Method: Because the magnet is provided with a shielding plate for protection from heat, a heatable quartz cell can now be placed in the center of the magnet. So, a sample can be measured by the Zeeman correction method. (Stable data is available with noise and drift corrected. See Data 2.)
3. INSTRUMENT
The Hitachi Model Z-2000 polarized Zeeman
atomic absorption spectrophotometer was used for the measurement. The Model
Z-2000 adopts the Zeeman effect-based background correction method which is
reliable even with a high background absorption.
4. PRETREATMENT
0.5 g of each foodstuff sample was injected into a Teflon decomposition container for the microwave digestion oven. 5 mL of nitric acid and 2 mL of hydrogen peroxide water were added, and then the container was fixed in the holder. A heating program was run (for about half an hour).
After returning the decomposition container
to room temperature by cooling water, the content was transferred into a
plastic volumetric flask of 25 mL and adjusted to the determined volume using
ultrapure water. The resulting solution was measured.
5. MEASUREMENT
Added for potassium iodide and hydrochloric acid were added for a 15-minute preliminary reduction. This solution was injected into the hydride formation system and measured by working curve method. The analytical result is shown in the following data sheet.
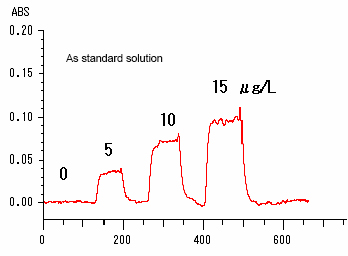
Single Beam
Method (Data 1)
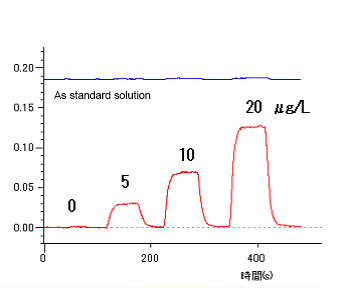
Zeeman Correction Method (Data 2)
Fig. 1 Model Z-2000 Polarized Zeeman Atomic Absorption Spectrophotometer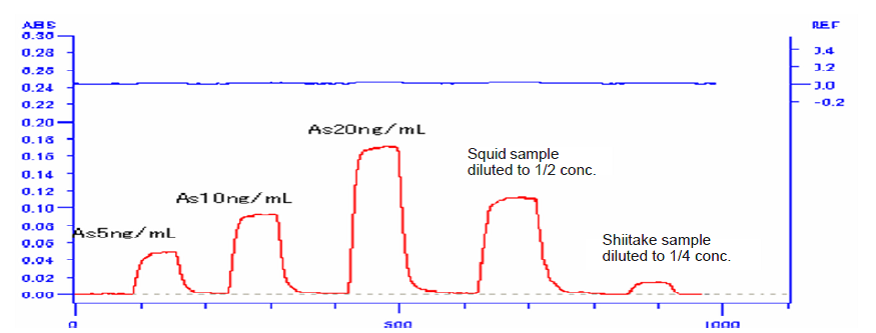
Time (s)
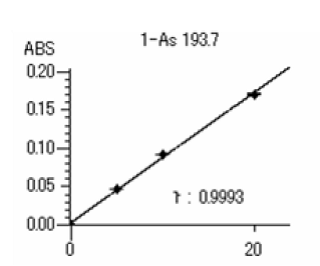
Concentration (ng/mL)
As
|
Measurement signal : |
Background correction |
|
Measuring wavelength (nm) : |
193.7 |
|
Slit width (nm) : |
1.3 |
|
Lamp current (mA) : |
12.0 |
|
Signal calculation : |
Integration |
|
Wavelength setting method : |
Automatic |
|
Time constant (s) : |
2.0 |
|
Photomultiplier voltage (V) : |
46.2 |
Analytical Conditions
1-As
|
Atomizer : |
Standard burner |
|
Fuel gas flow rate (L/min) : |
1.0 |
|
Supporting gas flow rate (L/min) : |
15.0 |
|
Delay time (s) : |
5 |
|
Kind of flame : |
Air-C2H2 |
|
Supporting gas pressure (kPa) : |
160 |
|
Burner height (mm) : |
7.5 |
|
Data sampling time (s) : |
5.0 |



 2606, 26/F., Tower 1, Ever Gain Plaza, 88 Container Port Road, Kwai Chung, N.T., Hong Kong
2606, 26/F., Tower 1, Ever Gain Plaza, 88 Container Port Road, Kwai Chung, N.T., Hong Kong +852-27519488 / WhatsApp/WeChat HK: +852-8491 7250
+852-27519488 / WhatsApp/WeChat HK: +852-8491 7250 techcomp@techcomp.com.hk
techcomp@techcomp.com.hk
 Sweep The Concern Us
Sweep The Concern Us