Home· · ·Techcomp college· · ·Application notes
Application notes
Acidity and vitamin C in citrus juice
Acidity and vitamin C in citrus juice by acid/base and redox titrations
Chemical Structure of Vitamin C
Vitamin C, also known as ascorbic acid and L-ascorbic acid,
is a vitamin found in food and used as a dietary supplement. The disease scurvy is prevented and treated
with vitamin C-containing foods or dietary supplements. Vitamin C is an essential nutrient involved
in the repair of tissue and the enzymatic production of certain
neurotransmitters. It is required for
the functioning of several enzymes and is important for immune system function. It also functions as an
antioxidant. Foods containing vitamin C
include citrus fruits, broccoli, Brussels sprouts, raw bell peppers, and
strawberries. Prolonged storage or
cooking may reduce vitamin C content in foods.
Acidity and vitamin C in refreshing drinks are titrated
successively to quantify each component.
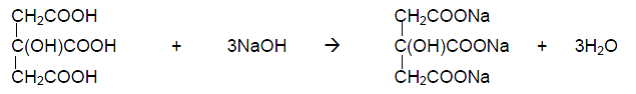
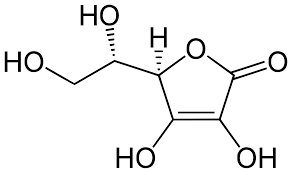
First, citric acid in the sample is titrated with sodium hydroxide using glass/reference combination electrode.
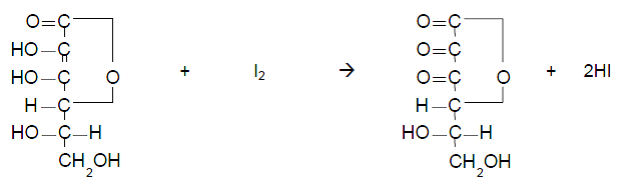
When titration of citric acid is completed, acetic acid is added to acidify the sample. The indicator electrode is switched to platinum electrode automatically and vitamin C is titrated with iodine
More Hiranuma
In this method, two different components are analyzed successively using two types of indicator electrodes and titrants. While this method used iodine titration method for determining vitamin C, there is an alternative method called “indophenol method”. It must be noted that indophenol method may be specified depending on the type of samples.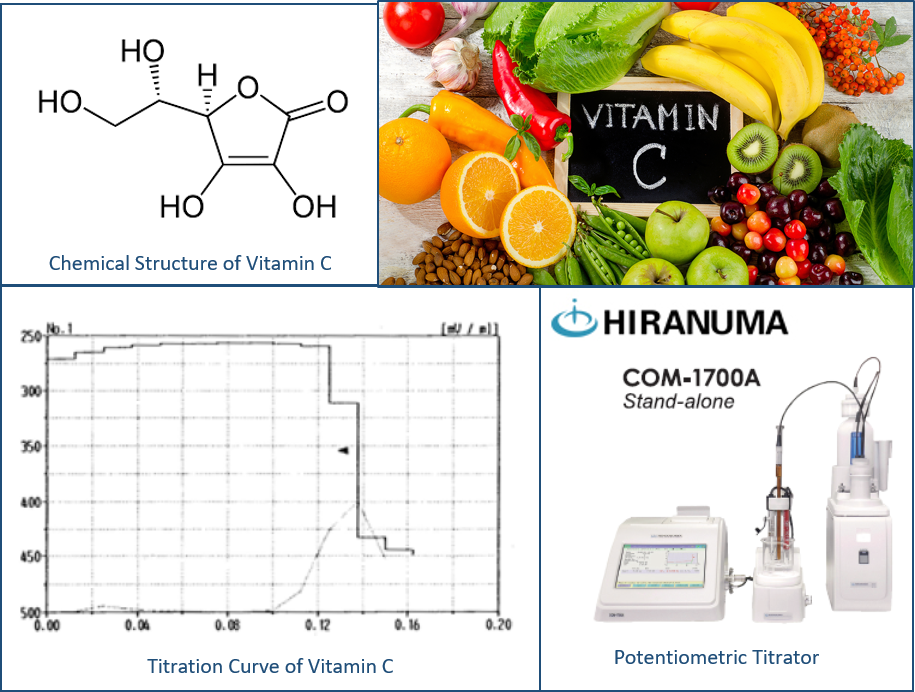
About Hiranuma COM-1700A Potentiometric Titrator

Website: http://www.hiranuma.com/english/index.html
More about Vitamin C
Wikipedia: https://en.wikipedia.org/wiki/Vitamin_C
More about Titration
Wikipedia: https://en.wikipedia.org/wiki/Titration



 2606, 26/F., Tower 1, Ever Gain Plaza, 88 Container Port Road, Kwai Chung, N.T., Hong Kong
2606, 26/F., Tower 1, Ever Gain Plaza, 88 Container Port Road, Kwai Chung, N.T., Hong Kong +852-27519488 / WhatsApp/WeChat HK: +852-8491 7250
+852-27519488 / WhatsApp/WeChat HK: +852-8491 7250 techcomp@techcomp.com.hk
techcomp@techcomp.com.hk
 Sweep The Concern Us
Sweep The Concern Us